Guidance for the Installation and Maintenance of Bed Rails
The correct installation and maintenance of bed rails is of major importance to a SNF’s ability to adhere to the rules specified by the FDA and the United States Consumer Product Safety Commission (CPSC) to prevent injuries from entrapment.
The FDA and CPSC have together provided the following guidelines with regard to bed rails:
– Before bed rails are installed, the facility should check with the manufacturer to make sure all the components (the bed rails, mattress, and bed frame) are compatible with each other.
– Rails should not be placed in such a way that they lead to a resident being forced to climb over them to get in and out of bed.
– In this regard, care must be taken to ensure that the bed’s dimensions are appropriate for the resident, and that the rails are appropriate for the size and weight of the user.
– The mattress and bed rails must be regularly inspected for any possible areas of entrapment to adhere to good maintenance of bed rails. While there may be no such areas to begin with, body movement, mattress compression, or bed movement can create unexpected gaps over time. Entrapment can occur without the use of bed rails when there are gaps between the mattress and the bed frame or head and foot boards, or between the bed frame and the head and foot boards.
– Rails may also loosen over time due to bed movement, and they should also be inspected on a regular, and documented, basis.
Needless to say, any manufacturer equipment alerts and recalls have to be passed on to front line staff for further action.
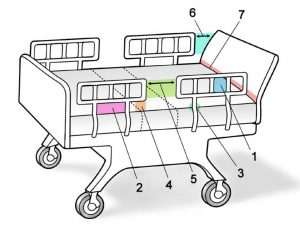
The FDA guidance has identified seven zones in a bed rail system as dangerous:
Zone 1: Within the Rail
Zone 2: Under the Rail, Between the Rail Supports or Next to a Single Rail Support
Zone 3: Between the Rail and the Mattress
Zone 4: Under the Rail, at the Ends of the Rail
Zone 5: Between Split Bed Rails
Zone 6: Between the End of the Rail and the Side Edge of the Head or Foot Board
Zone 7: Between the Head or Foot Board and the Mattress End.
In these seven zones, the FDA says there are three key body parts at risk for life-threatening entrapment: the head, neck, and chest.
To reduce the risk of head entrapment, openings in the bed system should not allow the widest part of a small head (head breadth measured across the face from ear to ear) to be trapped.
The FDA uses a head breadth dimension of 120mm (4¾ inches) as the basis for its dimensional limit recommendations.
To reduce the risk of neck entrapment, openings in the bed system should not allow a small neck to become trapped. The FDA uses a neck diameter dimension of 60mm (2⅜ inches) in this regard.
Finally, the openings in a bed system should be wide enough not to trap a large chest through the opening between split rails. The FDA uses a chest depth dimension of 318mm (12½ inches) as a standard.
See: “Guidance for Industry and FDA Staff: Hospital Bed System Dimensional and Assessment Guidance to Reduce Entrapment,” FDA, https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072729.pdf
Next: Avoiding Noncompliance Penalties